Abstract
Tyrosine kinase inhibitors (TKIs) have improved the outcomes of patients with Philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia (ALL). Many older patients (pts) are not good candidates for intensive chemotherapy and are treated with TKIs plus corticosteroids or low intensity chemotherapy. Although the remission rates with this approach have been high, the median disease-free survival (DFS) has been short. Therefore, novel treatment strategies are needed. This trial evaluated the feasibility of combining the TKI dasatinib with prednisone and blinatumomab in older pts with Ph+ ALL.
Methods: This trial was activated through the NCTN in January 2015 and closed to accrual in April 2021. Pt eligibility included: age ≥ 65 years; Ph+ or Ph-like ALL (with dasatinib-sensitive fusions or mutations); newly diagnosed or relapsed/ refractory; no evidence of central nervous system (CNS) disease; and adequate organ function.
Treatment: For induction, pts received dasatinib 140 mg/d orally (PO) Days 1-56 along with prednisone 60 mg/m 2/d PO Days 1-24. Pts achieving complete remission (CR) or CR with incomplete count recovery (CRi) (Day 28 or Day 56) continued dasatinib until Day 84 followed by 3 cycles of post-remission therapy (PRT) with blinatumomab/ dasatinib. Pts not achieving CR or CRi by Day 56 received re-induction with blinatumomab. Response was assessed at Day 35 of blinatumomab. Pts not achieving CR/ CRi could receive a second cycle of blinatumomab. This was followed by 3 cycles of PRT with blinatumomab/ dasatinib. Maintenance therapy consisted of prednisone 60 mg/m 2/d x 5 days every 28 days for a total of 18 cycles along with dasatinib 140 mg po qd indefinitely. CNS prophylaxis included intrathecal (IT) methotrexate every 4-6 weeks x 8 doses. IT methotrexate was given at least 2 days apart from blinatumomab.
Response was assessed at Days 28, 56, 84 and additional time points were dependent on response. Minimal residual disease (MRD) was assessed centrally by multi-color flow cytometry at Day 28.
Statistics: 9 eligible/ evaluable pts receiving PRT were to be evaluated before enrolling additional pts. Dose-limiting toxicities (DLTs) were defined as: > Grade 3 non-hematologic toxicities with the exception of nausea, vomiting, or diarrhea (if manageable with supportive care measures) or Grade 4 neutropenia lasting > 42 days with possible relationship to dasatinib or blinatumomab. If due to unexpected accrual, 12 pts were evaluable for DLT, the following rules would apply. If > 4 of the 12 pts experienced a DLT, the study would be temporarily closed pending review. Upon re-opening, 8 additional eligible and evaluable pts would be enrolled for a total of 20 pts receiving blinatumomab.
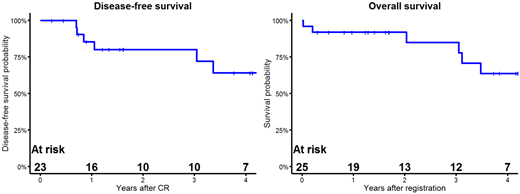
Results: Due to rapid accrual, 16 pts enrolled before accrual was paused to assess feasibility and safety. Twelve pts were evaluable for DLT and 4 experienced a DLT: Grade 3 dyspnea and gastrointestinal pain (n=1), Grade 3 hypertension (n=1), Grade 3 dyspnea (n=1), Grade 3 hyperglycemia (n=1). These adverse events were deemed acceptable by the NCI and FDA and the study re-opened. A total of 25 eligible pts were accrued. The median age was 73 years (range 62-87). All pts were newly diagnosed. One pt was Ph-like, with the remainder being Ph+; 89% of Ph+ pts had additional cytogenetic abnormalities. During induction, 2 pts experienced treatment-related non-hematologic Grade 4 toxicities. No Grade 4 or higher treatment-related non-hematologic toxicities occurred during post-remission therapy or maintenance. Twenty-three out of 25 pts (92%) achieved a CR during dasatinib-based and prednisone induction therapy. Four did not receive PRT (2 due to adverse events, 1 to receive transplant, 1 because of insurance issues). Sixteen pts who achieved CR had MRD data. Five out of 16 pts (31%) were MRD negative at Day 28. One pt remains on PRT and 10 are on maintenance. The median follow up for pts who are alive is 1.7 years. The median overall survival (OS) and DFS have not been reached as of July 8, 2021. Kaplan-Meier 3-year estimates of OS and DFS are 85% (95% CI 58%-95%) and 80% (95% CI 55%-92%), respectively (Figure).
Conclusions: This trial demonstrates the feasibility of combining dasatinib and blinatumomab in Ph+ ALL. In addition, with 1.7 years of follow up, outcomes are encouraging with high estimated 3-year DFS and OS. Longer follow up will be needed to determine the durability of these results.
Advani: Kite Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Research Funding; Glycomimetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Immunogen: Research Funding; OBI: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Research Funding; Macrogenics: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Moseley: BioSight Ltd: Consultancy. Wood: Pfizer, Amgen, Seattle Genetics: Honoraria; Juno, Pfizer, Amgen, Seattle Genetics: Other: Laboratory Services Agreement. Park: Intellia: Consultancy; Kura Oncology: Consultancy; BMS: Consultancy; Affyimmune: Consultancy; Curocel: Consultancy; Novartis: Consultancy; Artiva: Consultancy; PrecisionBio: Consultancy; Servier: Consultancy; Kite Pharma: Consultancy; Innate Pharma: Consultancy; Minerva: Consultancy; Autolus: Consultancy; Amgen: Consultancy. Wieduwilt: Gilead: Membership on an entity's Board of Directors or advisory committees; Reata: Current holder of stock options in a privately-held company. Jeyakumar: Jazz: Research Funding; Pfizer: Research Funding. Atallah: Abbvie: Consultancy, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Takeda: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Amgen: Consultancy. Gerds: PharmaEssentia Corporation: Consultancy; CTI BioPharma: Research Funding; Sierra Oncology: Consultancy; AbbVie: Consultancy; Celgene/Bristol Myers Squibb: Consultancy; Constellation: Consultancy; Novartis: Consultancy. O'Brien: Amgen, Astellas, Celgene, GlaxoSmithKline, Janssen Oncology, Aptose Biosciences Inc., Vaniam Group LLC, AbbVie, Alexion, Verastem, Juno Therapeutics, Vida Ventures, Autolus, Johnson and Johnson, Merck, Bristol Myers Squibb, NOVA Research Company, Eli Lill: Consultancy; Kite, Regeneron, Acerta, Caribou, Gilead, Pharmacyclics, TG Therapeutics, Pfizer, Sunesis: Research Funding. Othus: Celgene: Other: Data safety monitoring board; Merck: Consultancy; Biosight: Consultancy; Glycomimetics: Other: Data safety monitoring board; Daiichi Sankyo: Consultancy. Litzow: Jazz: Other: Advisory Board; Pluristem: Research Funding; Amgen: Research Funding; Actinium: Research Funding; Omeros: Other: Advisory Board; Astellas: Research Funding; AbbVie: Research Funding; Biosight: Other: Data monitoring committee. Stone: Actinium: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy; Arog: Consultancy, Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees; BerGen Bio: Membership on an entity's Board of Directors or advisory committees; Boston Pharmaceuticals: Consultancy; Bristol Myers Squibb: Consultancy; Elevate Bio: Membership on an entity's Board of Directors or advisory committees; Foghorn Therapeutics: Consultancy; Gemoab: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy; Innate: Consultancy; Janssen: Consultancy; Jazz: Consultancy; Novartis: Consultancy, Research Funding; Onconova: Consultancy; Syndax: Membership on an entity's Board of Directors or advisory committees; Syntrix/ACI: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; Aprea: Consultancy; Amgen: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Agios: Consultancy, Research Funding; Celgene: Consultancy; Macrogenics: Consultancy. Erba: AbbVie Inc; Agios Pharmaceuticals Inc; ALX Oncology; Amgen Inc; Daiichi Sankyo Inc; FORMA Therapeutics; Forty Seven Inc; Gilead Sciences Inc; GlycoMimetics Inc; ImmunoGen Inc; Jazz Pharmaceuticals Inc; MacroGenics Inc; Novartis; PTC Therapeutics: Research Funding; AbbVie Inc; Agios Pharmaceuticals Inc; Bristol Myers Squibb; Celgene, a Bristol Myers Squibb company; Incyte Corporation; Jazz Pharmaceuticals Inc; Novartis: Speakers Bureau; AbbVie Inc: Other: Independent review committee; AbbVie Inc; Agios Pharmaceuticals Inc; Astellas; Bristol Myers Squibb; Celgene, a Bristol Myers Squibb company; Daiichi Sankyo Inc; Genentech, a member of the Roche Group; GlycoMimetics Inc; Incyte Corporation; Jazz Pharmaceuticals Inc; Kura Oncology; Nov: Other: Advisory Committee.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal